Medical devices - Post-market surveillance for manufacturers
Preview in attachment
As medical devices are designed, developed, manufactured and distributed on the global market, a residual risk with regard to the medical device’s safety and performance remains throughout the product life cycle. This is due to a combination of factors, such as product variability, factors affecting the medical device’s use environment, the different end user interaction, as well as unforeseen medical device failure or misuse. Design and development activities of medical devices ensure that the residual risk is acceptable before product release (i.e. pre-market).
However, it is important to collect and analyse information on the medical device during production and post-production to meet requirements for monitoring of product and processes and ensure the residual risk remains acceptable. Appropriate processes for collecting and analysing the information on the production and post-production feedback allows for early detection of any undesirable effects. These processes can also reveal opportunities for improvement, as specified in ISO 13485, or possible relevance to safety, as specified in ISO 14971.
Post-market surveillance is the process to enable manufacturers to perform such monitoring, by collecting data from actual use of medical devices, analysing these data and then using the information from post-market surveillance in the appropriate processes, such as product realization, risk management, communicating to regulatory authorities or product improvement. The extent of a post-market surveillance process needs to be appropriate and proportionate to the medical device and its use.
The intent of this document is to provide guidance to manufacturers who are planning and executing their post-market surveillance activities. Other organizations, such as importers, distributors and reprocessors, that are connected to the manufacturer in the product lifecycle and who play a role in post-market surveillance activities, can also utilize the guidance in this document for their activities. In the rest of this document, the term organization will be used instead of manufacturer, as far as applicable.
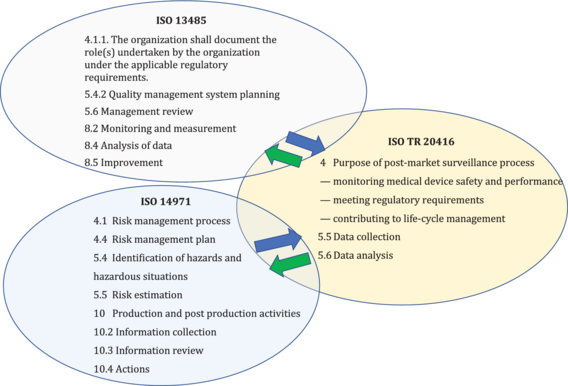
The guidance on the post-market surveillance process described in this document is complimentary to requirements in ISO 13485 and ISO 14971 for production and post-production activities to conduct post-market surveillance, see Figure 1.
Key
 |
setting requirements |
 |
provide deliverables |
Figure 1 - Inter-relationship of ISO TR 20416 with ISO 13485 and ISO 14971 standards
...
Decisions and actions, based on the information collected and analysed by application of this document, are described in other standards, such as ISO 13485 and ISO 14971, and are therefore not included in this document. The organization may be required to perform post-market surveillance activities to fulfil applicable regulatory requirements for medical devices. While regulatory requirements are not described here, this document can be helpful for organizations in fulfilling those regulatory requirements.
This document uses the definition of post-market surveillance from ISO 13485. Users of this document should note that the use of terms with respect to post-production data can vary in different jurisdictions and define different activities and responsibilities, for example market surveillance.
...
Allegati
|
Descrizione |
Lingua |
Dimensioni |
Downloads |
 |
|
EN |
132 kB |
12 |