// Documenti disponibili n: 46.583
// Documenti scaricati n: 36.633.365

In order to ensure a coherent application of the Pressure Equipment Directive 97/23/EC (PED), Guidelines are developed and agreed by the Commission's Working Group "Pressure" (WGP).
This working group, created as a result of Article 17 of the PED, which requests the Member States to cooperate in order to assist the functioning of this Directive, is composed of representatives of Member States, European federations, the Notified Bodies Forum and CEN and chaired by a representative of the Commission services.
Document history
Version Date Comment
1.0 1/12/2010 Includes PED Guidelines up to WGP meeting of 24/11/2010
1.1 19/7/2011 Quality check, correction of links, introduction
1.2 30/8/2011 Quality check
1.3 20/03/2012 Includes PED Guidelines up to WGP meeting of 06/03/2012
1.4 26/03/2013 Includes PED Guidelines up to WGP meeting of 07/03/2013
1.5. 4/04/2014 Includes PED Guidelines up to WGP meeting of 22/03/2014
Agg. 04.2014 V. 1.5
Commission's Working Group "Pressure" WGP

Redazione e validazione
La redazione del Manuale di Istruzioni di una macchina è un obbligo che il Fabbricante deve assolvere secondo le indi...

Update November 2017
Frequently Asked Questions (FAQ) on the Ecodesign Directive 2009/125/EC establishing a framework for the setting of e...
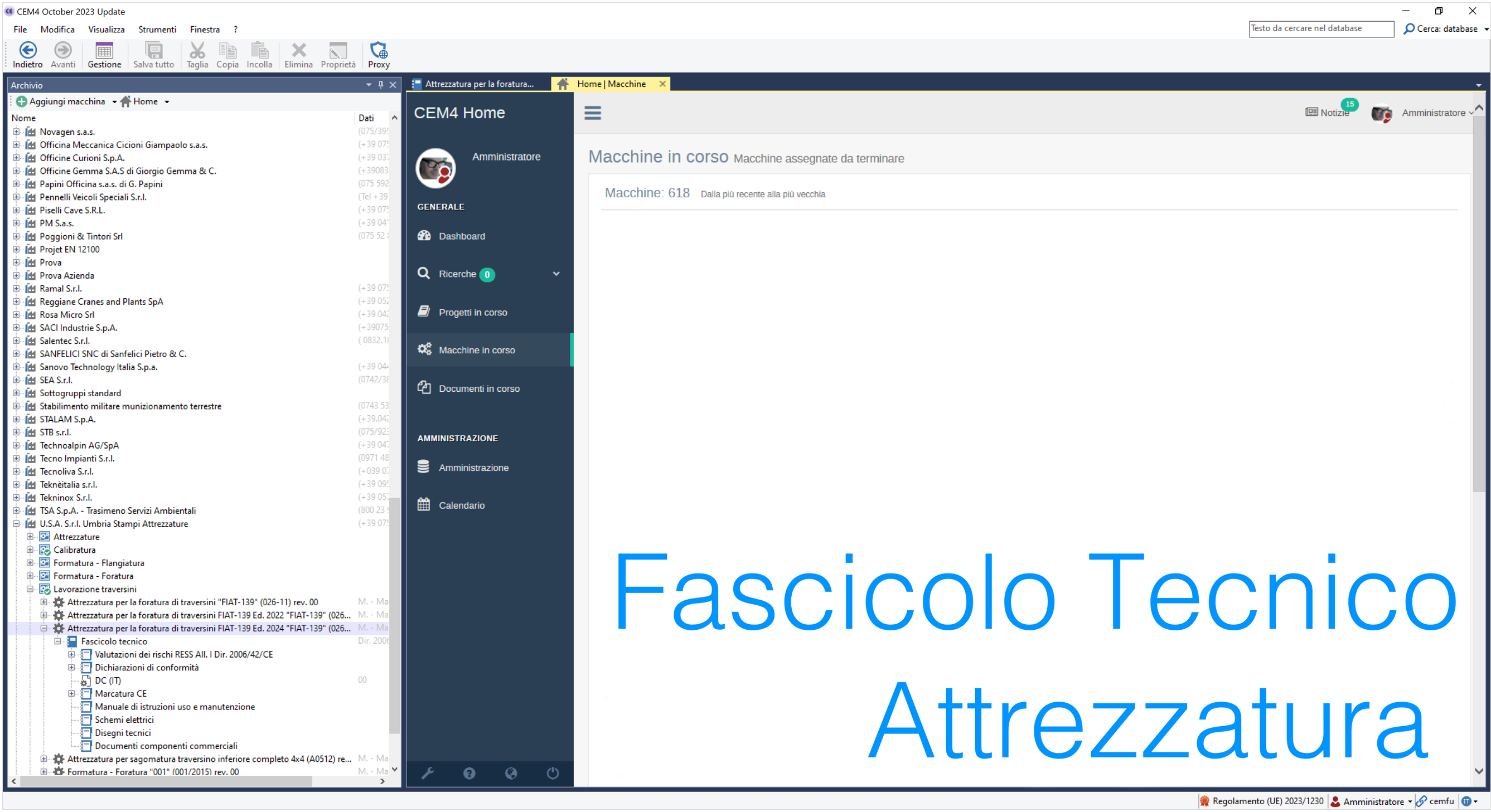
ID 1541 | 22.04.2024 Ed. 2024 / Documenti Fascicolo Tecnico allegati
Esempio di Fascicolo Tecnico realizzato con CEM4
I...
Testata editoriale iscritta al n. 22/2024 del registro periodici della cancelleria del Tribunale di Perugia in data 19.11.2024