// Documenti disponibili n: 46.322
// Documenti scaricati n: 36.174.428
MDCG 2019-14 Explanatory note on MDR codes
Commission Implementing Regulation 2017/2185 establishes the codes for the designation of notified bodies in medical devices under Regulation (EU) 2017/745 and in vitro diagnostic medical devices under Regulation (EU) 2017/746.
These codes are primarily used by designating authorities to define the notified body scope of designation but they are also used by the notified body to:
These codes may be very broad and, furthermore, unequivocal authorisation of personnel to codes and the assignment of codes to a device is not always straightforward. However, the notified body’s system needs to ensure, in all cases, that the authorisation of personnel and team allocation for the conformity assessment of a device ensures adequate knowledge and expertise.
The lists of codes and corresponding types of devices established by the above mentioned Regulation takes into account various device types which can be characterised by design and intended purpose, manufacturing processes and technologies used, such as sterilisation and the use of nanomaterials.
These lists of codes should be used in a way that provides for a multi-dimensional application to all typology of devices. This will ensure that notified bodies as well as the staff assigned to conformity assessment are fully competent for the devices they are required to assess.
This guidance is intended to explain the different level of codes and how they should be used, including the use of conditions with a view to ensure a harmonised use of the codes especially for the allocation of resources to conformity assessment activities.
Fonte: EU
Collegati:

Report 34 del 26/08/2016 N.2 A12/1071/16 Ungheria
Approfondimento tecnico: Seggiolino da bicicletta per bambini
Il prodotto, di marca Kross/bg, Mo...

Altre Direttive applicabili:
Direttiva Bassa tensione 2006/95/CE
Direttiva Compatibilità Elettromagnetica 2004/108/CE
[Elaborazio...
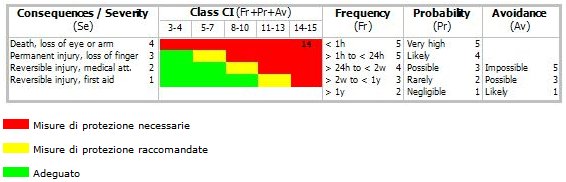
Sicurezza macchine: Stima del Rischio
Procedura per la Stima del Rischio in accordo con la
Norma Tecnica/Technical Report ISO/TR 14121-2 ...
Testata editoriale iscritta al n. 22/2024 del registro periodici della cancelleria del Tribunale di Perugia in data 19.11.2024