// Documenti disponibili n: 46.570
// Documenti scaricati n: 36.611.237
Diagnostic devices using non-ionizing radiation: existing regulations and potential health risks international commission on non-ionizing radiation protection
Use of non-ionizing radiation (NIR) for diagnostic purposes allows non-invasive assessment of the structure and function of the human body and is widely employed in medical care.
ICNIRP has published previous statements about the protection of patients during medical magnetic resonance imaging (MRI), but diagnostic methods using other forms of NIR have not been considered.
This statement reviews the range of diagnostic NIR devices currently used in clinical settings; documents the relevant regulations and policies covering patients and health care workers; reviews the evidence around potential health risks to patients and health care workers exposed to diagnostic NIR; and identifies situations of high NIR exposure from diagnostic devices in which patients or health care workers might not be adequately protected by current regulations.
Diagnostic technologies were classified by the types of NIR that they employ. The aim was to describe the techniques in terms of general device categories which may encompass more specific devices or techniques with similar scientific principles. Relevant legally-binding regulations for protection of patients and workers and organizations responsible for those regulations were summarized.
Review of the epidemiological evidence concerning health risks associated with exposure to diagnostic NIR highlighted a lack of data on potential risks to the fetus exposed to MRI during the first trimester, and on long-term health risks in workers exposed to MRI. Most of the relevant epidemiological evidence that is currently available relates to MRI or ultrasound. Exposure limits are needed for exposures from diagnostic technologies using optical radiation within the body.
There is a lack of data regarding risk of congenital malformations following exposure to ultrasound in utero in the first trimester and also about the possible health effects of interactions between ultrasound and contrast media.
International Commission on Non-Ionizing Radiation Protection (ICNIRP)
2017

INAIL, 2021
Partendo dal patrimonio informativo che negli anni l’Istituto ha costituito e dalle competenze maturate nell’espletamento de...
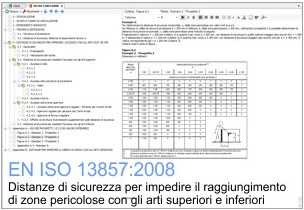
Distanze di sicurezza per impedire il raggiungimento di zone pericolose con gli arti superiori e inferiori
La presente norma internazionale sta...

Il 10° rapporto si propone come strumento...
Testata editoriale iscritta al n. 22/2024 del registro periodici della cancelleria del Tribunale di Perugia in data 19.11.2024