// Documenti disponibili n: 46.709
// Documenti scaricati n: 36.901.288
ID 19311 | 26.03.2023 / Preview in allegato
ISO 14708-1:2014
Implants for surgery - Active implantable medical devices - Part 1: General requirements for safety, marking and for information to be provided by the manufacturer
Data pubblicazione: 2014
_______
ISO 14708-1:2014 specifies requirements that are generally applicable to active implantable medical devices. The tests that are specified in ISO 14708 are type tests and are to be carried out on samples of an active implantable medical device to show compliance. ISO 14708-1:2014 is applicable not only to active implantable medical devices that are electrically powered but also to those powered by other energy sources (for example by gas pressure or by springs).
ISO 14708-1:2014 is also applicable to some non-implantable parts and accessories of the active implantable medical devices.
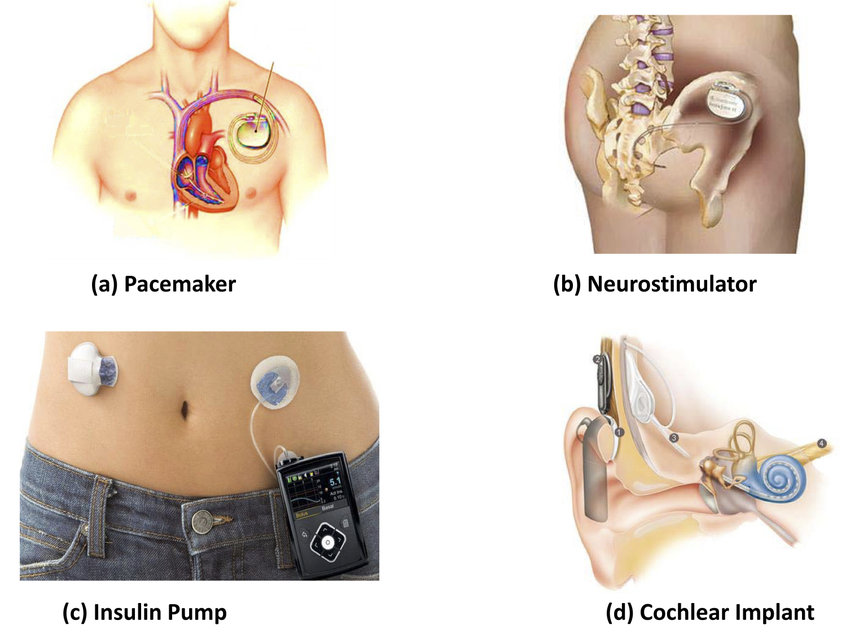
Example Active implantable medical devices
Collegati

ID 20949 | 11.12.2023 / In allegato
UNI/PdR 51:2018
Responsabilità sociale nelle Micro e Piccole I...

Imminente la pubblicazione delle nuove edizioni: sono aperte le prenotazioni
Il 1° dicembre 2015 è stata pubblicata la nuova revisione della norma UNI 7129,...

ID 8678 | 06.07.2019
ISO 20607:2019
Safety of machinery - Instruction handbook - General drafting principles
Vedi Documento ISO 20607:2019 IT
1a Ed. ...
Testata editoriale iscritta al n. 22/2024 del registro periodici della cancelleria del Tribunale di Perugia in data 19.11.2024