// Documenti disponibili n: 46.452
// Documenti scaricati n: 36.403.802
ID 19311 | 26.03.2023 / Preview in allegato
ISO 14708-1:2014
Implants for surgery - Active implantable medical devices - Part 1: General requirements for safety, marking and for information to be provided by the manufacturer
Data pubblicazione: 2014
_______
ISO 14708-1:2014 specifies requirements that are generally applicable to active implantable medical devices. The tests that are specified in ISO 14708 are type tests and are to be carried out on samples of an active implantable medical device to show compliance. ISO 14708-1:2014 is applicable not only to active implantable medical devices that are electrically powered but also to those powered by other energy sources (for example by gas pressure or by springs).
ISO 14708-1:2014 is also applicable to some non-implantable parts and accessories of the active implantable medical devices.
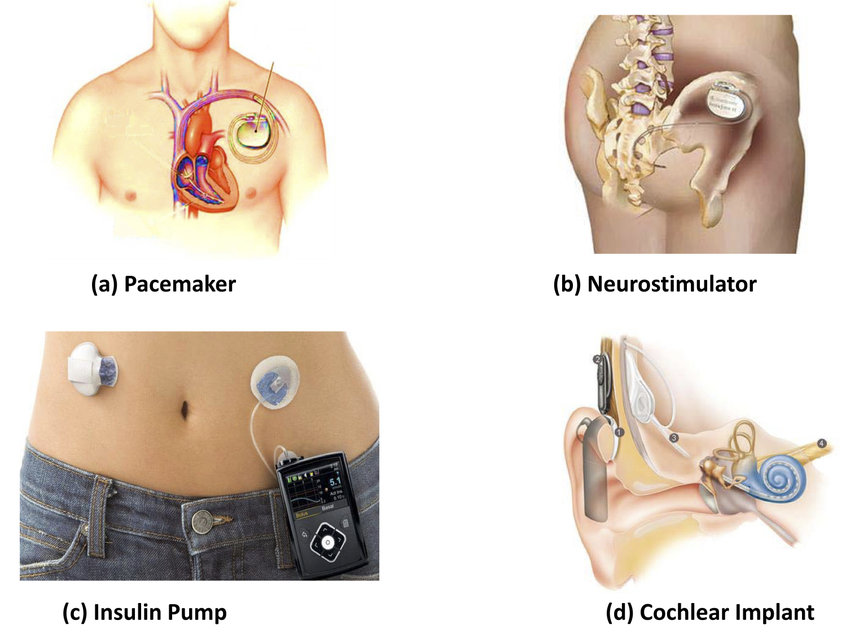
Example Active implantable medical devices
Collegati

ID 16430| 19.04.2022 / Allegata Preview
CEI EN IEC 61439-1:2022 Apparecchiature assiemate di protezione e di manovr...

UNI EN 12331:2021 Macchine per l'industria alimentare - Macchine tritacarne - Requisiti di sicurezza e di igiene
Recepisce: EN 12331:2021
Data entrata in vigore: 04 no...

ID 21946 | 29.05.2024 / Preview attached
Fire safety engineering - Design of evacuation experiments
_______
This document specifies a meth...
Testata editoriale iscritta al n. 22/2024 del registro periodici della cancelleria del Tribunale di Perugia in data 19.11.2024