// Documenti disponibili n: 46.261
// Documenti scaricati n: 35.998.765

This guidance is intended to describe the Food and Drug Administration's (FDA’s) current thinking regarding the scope and application of part 11 of Title of the Code of Federal Regulations; Electronic Records; Electronic Signatures (21 CFR Part 11).
This document provides guidance to persons who, in fulfillment of a requirement in a statute or another part of FDA's regulations to maintain records or submit information to FDA, have chosen to maintain the records or submit designated information electronically and, as a result, have become subject to part 11.
Part 11 applies to records in electronic form that are created, modified, maintained, archived, retrieved, or transmitted under any records requirements set forth in Agency regulations. Part 11 also applies to electronic records submitted to the Agency under the Federal Food, Drug, and Cosmetic Act (the Act) and the Public Health Service Act (the 29 PHS Act), even if such records are not specifically identified in Agency regulations (§ 11.1).
The underlying requirements set forth in the Act, PHS Act, and FDA regulations (other than part 31 11) are referred to in this guidance document as predicate rules.
FDA
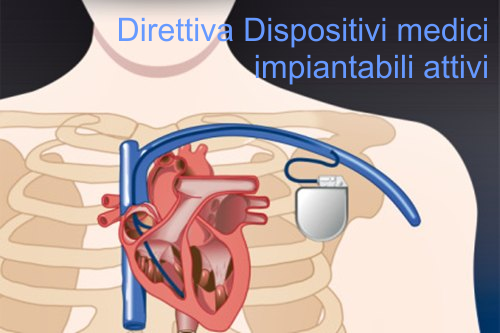
Comunicazione della Commissione nell’ambito dell’applicazione della direttiva 90/385/CEE del Consiglio, del 20 gi...

ID 24007 | 22.05.2025 / In allegato Preview
UNI EN ISO 16089:2025 Macchine utensili - Sicurezza - Rettificatrici fisse
La norma specifica i requisiti e/o le ...

Adeguamento della normativa nazionale alle disposizioni del regolamento (UE) n. 1025/2012 del Parlamento europeo e del Consiglio, del 25 ottobre 2012, sulla ...
Testata editoriale iscritta al n. 22/2024 del registro periodici della cancelleria del Tribunale di Perugia in data 19.11.2024